Solar Disinfection of Drinking Water and Oral Rehydration Solutions
Home >
Resources >
Solar
Disinfection Guidelines for Household Application in Developing
Countries > Solar Energy: Some Practical Hints
Foreword
Oral Rehydration Therapy: The Revolution for Children
Oral Rehydration Therapy: The Four Simple Technologies
Global Rehydration Therapy: Global Diarrhoeal Diseases Control Programmes
Oral Rehydration Therapy: Causes, Transmission, and Control of Childhood Diarrhoea
Oral Rehydration Solutions: The Practical Issues
Oral Rehydration Solutions: Domestic Formulations
Oral Rehydration Solutions: Disinfection by Boiling
Solar Energy: Fundamental Considerations
Solar Energy: From Sun to Earth
Solar Energy: World Distribution
Solar Energy: A Competitor
Solar Energy: Some Practical Hints
Solar Disinfection Studies: Drinking Water
Solar Disinfection Studies: Oral Rehydration Solutions
Appendix: Source of Information on Diarrhoeal Diseases
Solar Energy
Some Practical Hints
Published Data
The only available data issued by most of the meteorological stations
is the total solar radiation (direct and diffuse radiations) received
on the surface of an object placed horizontally. Very few stations
provide data for the total radiation striking objects placed in a
vertical position.
For most solar radiation applications the available data may be
sufficiently adequate, but is not quite so for purposes of solar
disinfection of drinking water or oral rehydration solutions using
bottles or other similar containers. This is on account of two
important factors: (a) the effective component of solar radiation
involved in microbial destruction is in the near-ultraviolet (A) band
(300-400 nm), and to a lesser extent in the visible band of violet and
blue lights (400-490 nm); and (b) from a practical standpoint, bottles
or similar vessels used in the disinfection process must necessarily
be kept upright during exposure to sunlight. In this context,
therefore, some care must be taken in the interpretation of certain
published data that may not be pertinent to specific local situations
or applications. Ideally, each of the developing countries interested
in the development of programmes for the exploitation of solar
radiation should endeavour to establish solar research centres and
monitoring stations. This has been the trend in recent years in some
of the Arab states and other developing countries.
Seasonal Variations
It is logical to question the feasibility of utilizing solar energy
for any particular application in an effective manner throughout the
year without serious setbacks or interruptions caused by seasonal
variations in solar radiation. There is no doubt that seasonal
variations could provoke marked changes in the effectiveness or
productivity of solar dependent processes. For this reason it would be
useful to consider the possible variations and their potential
impacts.
Seasonal variations are primarily due to changes in the solar
altitude, and in cloud formation during the rainy season. These two
factors determine not only the total amount of solar radiation
reaching ground-level at a given location, but also the proportion of
the various kinds of radiation. As a general rule, the lower the sun
is with reference to the horizon the weaker is the total solar
radiation, and the greater is the fraction of scattered light, mainly
in the UV (A) and blue light bands. The reverse is also true when the
solar altitude increases. Cloud formation may hinder the overall
atmospheric transmission of solar radiation to a degree determined by
the thickness and density of clouds. Very dense clouds, about 1000 m
in thickness, are said to reflect back into space more than 90% of the
incident solar radiation. Such occurrences, however, are generally of
short duration in many parts of the world, and so their impact would
be transient. In any event, scattered radiation continues to retain
its destructive power against microorganisms, although it may be
somewhat attenuated.
In the northern hemisphere, for instance, nature decrees that during
the winter months the total solar radiation is much reduced, and the
length of the day becomes shorter. The lowest values occur in December
and January. From then on the values increase gradually, reaching the
highest levels in June and July. These facts are illustrated, at least
in part, in Figure 3 which is based on hourly measurements of solar
radiation at a wavelength peak of 357 n-m (an optimal radiation
wavelength for microbial destruction). The UV radiation measurements
were made in Beirut, Lebanon, on two cloudless days (October 8, 1983,
and December 21, 1983), using a Spectroline DM-357X digital radiometer
(obtained from Insect-O-Cutor Limited, Stockport, Cheshire, England).
The important inferences drawn from the two graphs in Figure 3
indicate that solar radiation at a wavelength of about 357 nm
decreases from October to December, and that the ultraviolet (A)
radiation reaches its maximum level at about noontime regardless of
the time of the year. In effect, this means that the microbial
disinfection process is expected to be much more efficient in summer
and autumn than in winter under clear sky conditions in Beirut. In
practice, therefore, lengthening the sunlight exposure period for
disinfection purposes during the winter months would accomplish the
desired results.
Orientation of Receiver
In solar operations using devices designed to collect or concentrate
solar radiation, it is generally advised to keep the radiation
receiver in a tilted position rather than in a horizontal position in
order to be at right angles to the sun's rays. The recommended angle
of inclination from the horizontal is equal to the latitude of the
location, and the receiver is to face the south. The amount of
sunlight collected and utilized is thus substantially increased, for
as much as 50% of solar energy could be gained, particularly in winter
when the sun is closer to the horizon.
The question that presents itself regarding the solar disinfection
process is whether a similar arrangement would be necessary and
justifiable. To arrive at a conclusion in this regard, it would be
necessary to evaluate the pertinent facts. For the usual solar
processes the aim is to collect on the receivers as much direct
sunlight as possible to attain an optimum efficiency. While this is
partly true for solar decontamination operations, it should be noted
that in these cases scattered radiation striking from all directions
an upright receiving object (e.g. bottle holding water to be
disinfected) presents an additional advantage. By tilting such an
object, which is a practical problem, more direct sunlight is gained
at the expense of scattered radiation.
 Figure 3.
Figure 3.
Solar radiation (near-ultraviolet-A)
on a horizontal target as a function of time.
Measurements made
in Beirut, Lebanon, on October 9, 1983 using a Spectroline digital
radiometer at a peak wavelength of 357 nm. Clear sky prevailed.
To help resolve this issue, comparative measurements were made of the
UV (A) radiation received on an object kept upright and then lying
horizontally. Readings were taken at intervals throughout the day
under clear sky conditions using the same radiometer described
earlier. The results shown graphically in Figure 4 lead to the
principal conclusion that an upright position is much more favourable
than a horizontal one. Other experimental data show that, with clear
skies, UV (A) radiation intensity values for vertical objects are
almost twice as great as those for horizontal objects throughout the
greater part of the day, and tend to become equal under conditions of
haze or cloudiness.
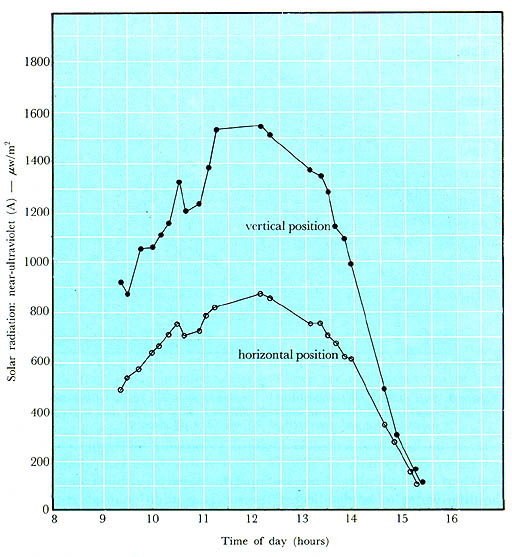 Figure 4.
Figure 4.
Effects of orientation of target on
solar radiation (near-ultraviolet-A) received
throughout the day.
Measurements made in Beirut, Lebanon, on January 1, 1984
using a
Spectroline radiometer at a peak wavelength of 357 nm. Clear sky
prevailed.
Transmission Through Glass
In selecting containers for solar disinfection of water or oral
rehydration solutions, the property of being transparent to sunlight
is of utmost importance. Because of their widespread availability,
glass containers should be considered with respect to transmission of
solar radiation at different wavelength bands, particularly with
respect to UV (A) radiation.
Ordinary glass of which most containers such as bottles and jars are
made can transmit solar radiation up to about 90%, the remainder being
reflected or absorbed by the glass. The amount and kind of radiation
that passes through ordinary glass depends on the colour and thickness
of the glass, and on the specific wavelength bands of radiation.
Colourless glass transmits solar radiation at wavelengths in the range
of 320 to 700 nm. It is therefore opaque to radiation below 320 nm,
and to infrared radiation. The maximum amount of radiation transmitted
occurs at 400 nm. Pyrex glass, of which most laboratory glassware is
made, is opaque to radiation below 280 nm, and attains a maximum
transmission at 340 nm. Transparent plastic materials such as
polystyrene and methylacrylate (Lucite and Plexiglass) can have
a higher radiation transmittance than glass at wavelengths greater
than 290 nm. These materials are therefore better than glass for the
transmission of germicidal solar radiation at wavelengths from 300 to
400 nm.
As for coloured glass, the commonest colouring agents are iron,
manganese, chromium, copper, and cobalt; but iron is the main
colouring material which gives glass a greenish tinge. Each of these
colourants imparts to glass a characteristic tint, and causes the
absorption of radiation at specific wavelengths. The iron content in
ordinary glass determines the transmission of solar radiation at
different wavelengths. Glass with a low iron content allows high
radiation transmittance at all wavelengths of the spectrum. For
wavelengths in the near-ultraviolet region (A) the transmittance is up
to about 90%. As the iron content increases and the glass attains a
darker green colour, the transmittance in the near-ultraviolet region
(A) decreases, hut remains at a fairly high level in the visible
region (400 to 700 nm).
With coloured glass, the tint perceived by the sight is due to the
specific wavelength of visible light transmitted through the
glass. For instance, blue glass appears to have a blue tint because
visible light in the blue band is transmitted much more than
others. Similarly, red glass transmits mostly visible light in the red
band of the spectrum. This is of importance in selecting the most
appropriate coloured glass containers for solar disinfection
purposes. Naturally, colourless glass with a low iron content would be
the best choice. Next comes the blue tinted glass.
Transmission Through Water
The discussion thus far has traced the fate of solar radiation as it
treverses the atmosphere to strike a target at ground-level, which is
assumed to be a glass or plastic container holding water or oral
rehydration solution. It should be clear by now that the most
effective germicidal component of solar radiation (300 to 400 nm)
reaching the target container and penetrating its walls remains
largely intact in terms of quantity, quality, and microbial
destructive action. What remains to be considered is the transmission
of the effective component of solar radiation through the water or
aqueous solutions to reach the ultimate target -- the microogranisms
to be destroyed.
That sunlight can penetrate water is a well known phenomenon. In fact,
it is an essential requirement to sustain the life of aquatic plants
like algae that grow in water. An assessment of solar radiation
transmission through colourless aqueous medium such as clear natural
water points out the fact that, as the penetration path gradually
increases, the radiation intensity decreases accordingly. The loss in
intensity varies with wavelength, being particularly low for
radiations of short wavelengths. For wavelengths ranging from 300 to
500 nm the reduction in intensity does not exceed 5% per metre of water
depth. For the higher wavelengths the value me be as high as 40% per
metre. The reduction at all wavelengths is largely due to radiation
scattering, for absorption by clear natural water constitutes only a
relatively small fraction. These facts show that UV (A) radiation will
penetrate clear water to a depth of several metres before it is
appreciably diminished in intensity. Obviously, then, UV (A) radiation
can be readily transmitted through small volumes of clear water
contained in transparent vessels. However, the picture differs in the
case of coloured or turbid water.
Substances imparting colour to water are likely to absorb radiation at
specific wavelengths that vary with the nature of the substance. Since
coloured waters are limited to highly polluted waters not fit for
drinking, such cases are of no relevance here. On the other hand,
suspended particles in water would cause radiation scattering by
deflection from their surfaces in all directions. this phenomenon is
known as the Tyndall effect. This can be easily demonstrated by
viewing a bottle with turbid water against a source of light. The
particles become visible, but not in the case of clear water or a
clear solution of salt, for instance. In view of this information,
water with a high content of suspended particles tends to obstruct the
passage of a beam of solar radiation, the penetration depth depending
upon the degree of turbidity of the water. In practice, this
phenomenon could be neglected if the water is only slightly turbid;
otherwise the turbidity needs to be reduced by allowing the larger
particles to settle or, better still, by filtration or coagulation of
the water.
|
